In this lecture we will learn what electric charge and current is and how electricity works. You can watch the following video or read the written tutorial below.
Structure of an Atom
To understand electricity, we need to start with the atom. Matter is made of atoms. We are made of atoms. Everything in the Universe is made of atoms.
An atom cannot be seen with a naked eye, so we’ll use a simplified model which can help us understand the structure of the atom. This is called the Bohr model.

In the center of the atom there is a nucleus, which consists of neutrons which have no charge, and protons which have a positive charge. Also, there are negatively charged electrons orbiting around the nucleus.
The number of neutrons, protons and electrons an atom has can tell us which material it is.
Shells
The protons and neutrons are positioned in the center of the atom, while the electrons circulate around the nucleus in а constant motion.
The electrons are much lighter than the protons in the nucleus, and they can move very easy with almost the speed of light.
They move around the nucleus in circular orbits, or shells. Each shell can contain only a particular number of electrons: The first shell can hold 2 electrons, the second shell 8 electrons, the third shell can hold up to 18, and so on.
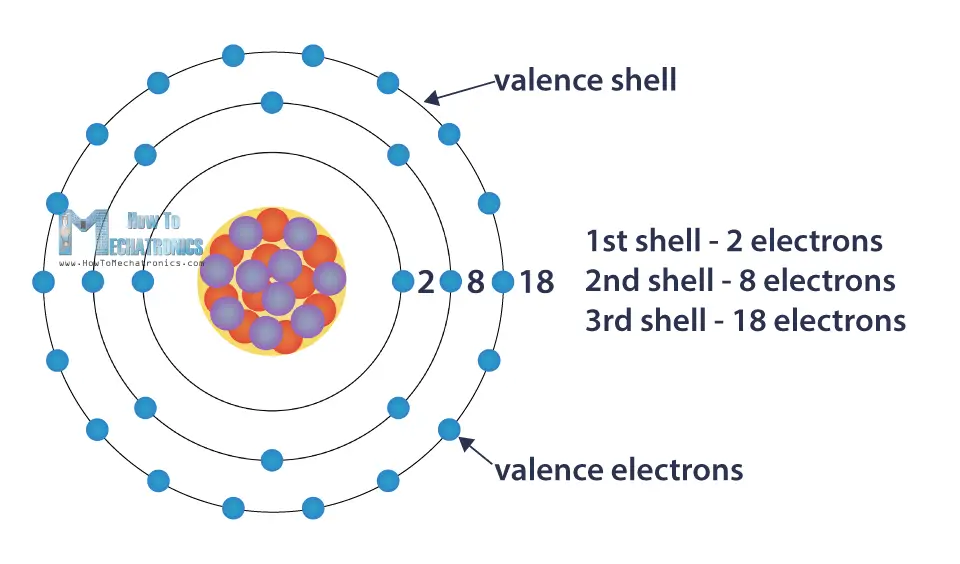
The shells are filled up with electrons from the inside out. The number of electrons in the last, outer shell determines the reactivity of the atom, or its tendency to form chemical bonds with other atoms. When that shell is full, the atom is stable and least reactive.
The outer shell is known as the valence shell, and the electrons found in it are called valance electrons. Some materials in this shell have loosely bound electrons which allows them to flow from one atom to another.
Conductors and Insulators
These moving electrons are called free electrons. How easy it is for electrons to move around depends on the material.
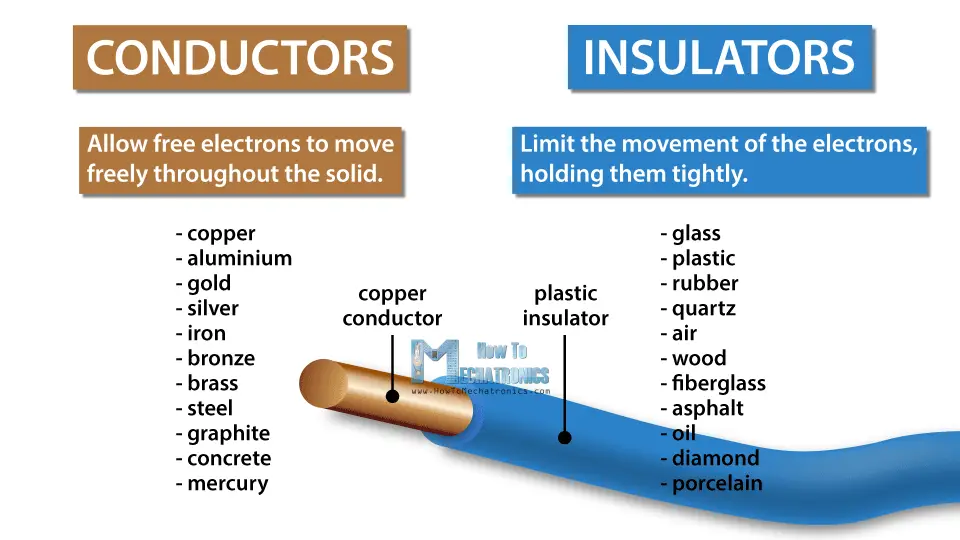
Materials can be either conductors or insulators. Materials that are conductors, like most metals, allow free electrons to move freely throughout the solid, while insulators, like plastic or glass, limit the movement of the electrons, holding them tightly.
Related: How a Capacitor Works – Capacitor Physics and Applications
Electric Charge
Generally, the atoms have neutral charge, which means they have the same number of electrons and protons. In other words, they have a net electric charge equal to zero. This is the lowest possible energy level of the atom, or so called the ground state.
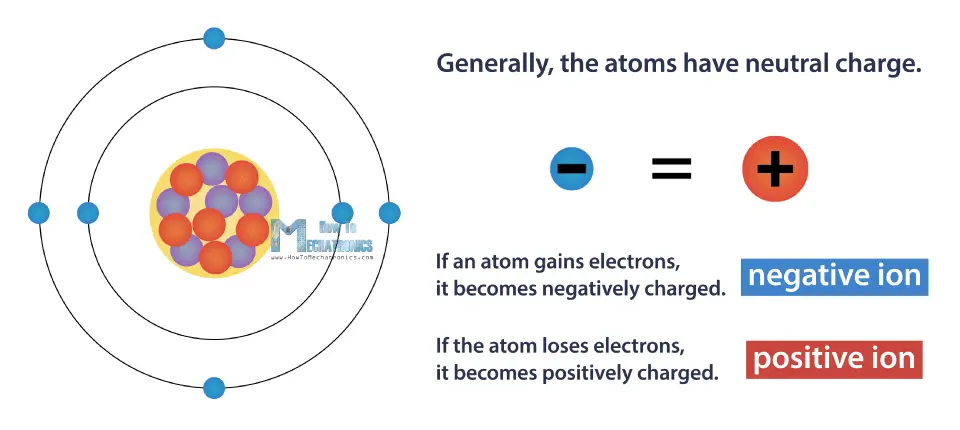
However, we can change the atom’s charge, by causing it to gain or lose electrons. If an atom gains electrons, it becomes negatively charged, and opposite, it the atom loses electrons, it becomes positively charged. Charged atom is called positive or negative ion.
Static Electricity
For example, when you drag your feet across a carpet, you are creating many surface contacts between your feet and the carpet, allowing electrons to transfer to you, thereby building up a static charge on your skin.
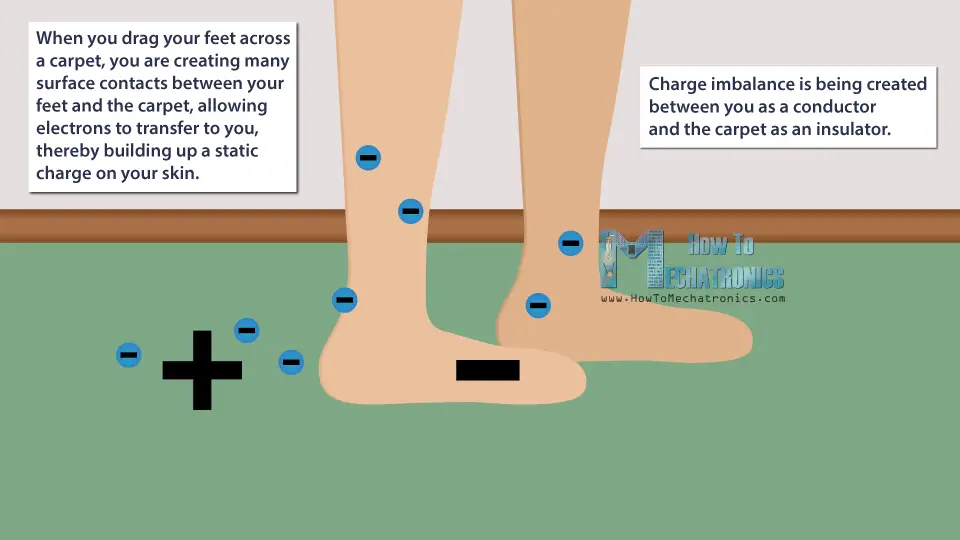
Instead of you and the carpet having a neutral charge, a charge imbalance is being created between you as a conductor and the carpet as an insulator.
Then, when you touch a door knob, which is metal, all the charge wants to leave you and go to the door knob in order to restore the charge imbalance. Therefore you get a shock as the electrons leave you.
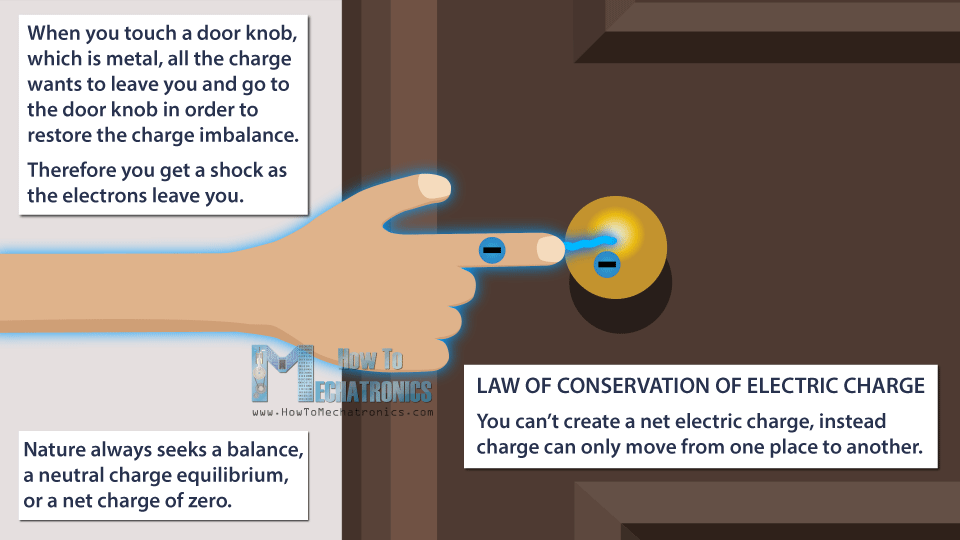
Nature always seeks a balance, a neutral charge equilibrium, or a net charge of zero. During this process we didn’t create new charges. The overall charge between the objects is still zero.
This leads us to the law of conservation of electric charge, which says that you can’t create a net electric charge, instead charge can only move from one place to another.
Electric charge in fact is the most important property of the protons and the electrons.
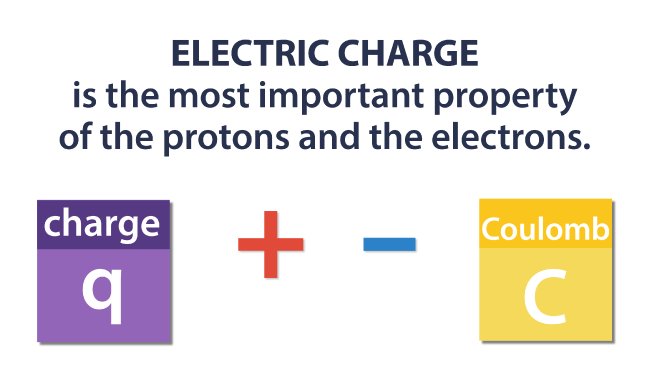
The charge is denoted by “q”, and the unit of charge is Coulomb. Objects can be positively or negatively charged, hence “q” can have both positive and negative values.
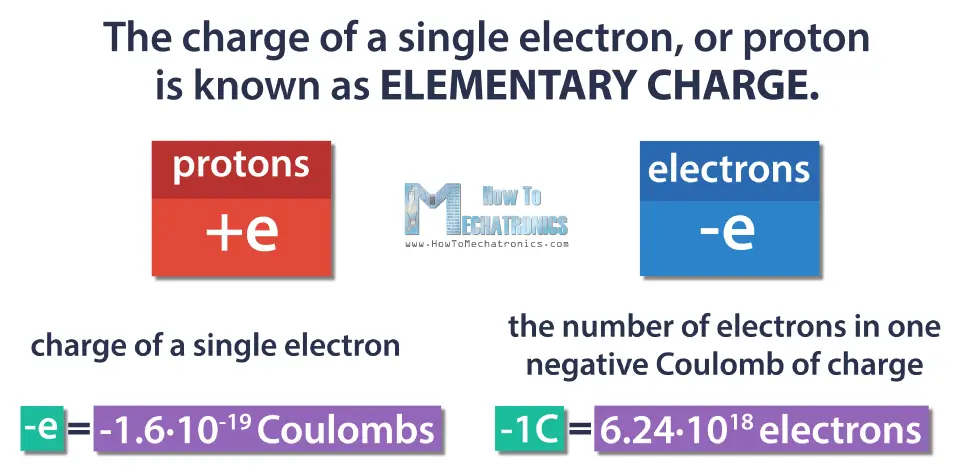
The charge of a single electron, or proton is known as elementary charge, and is denoted with a lowercase “e”. Protons have a charge of positive e, and electrons of negative e.
Electric Current
The flow of electrons is what forms an electric current.
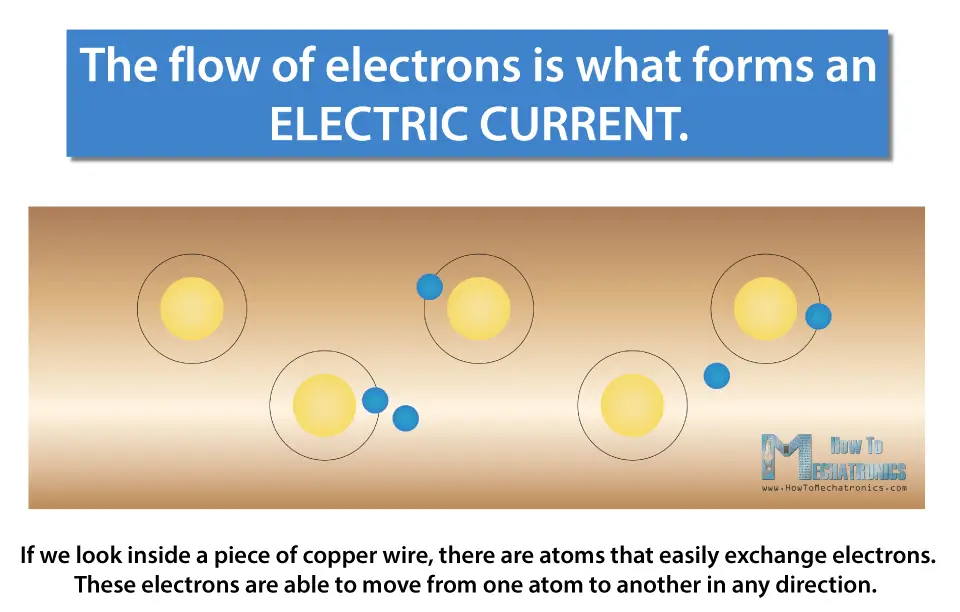
If we look inside a piece of copper wire, there are atoms that easily exchange electrons. These electrons are able to move from one atom to another in any direction. If we make a closed circuit, by connecting the copper wire with a power source, like a battery, then the voltage will make the electrons move in the same direction, from one terminal of the battery to the other, or from the negative to the positive of the power source.
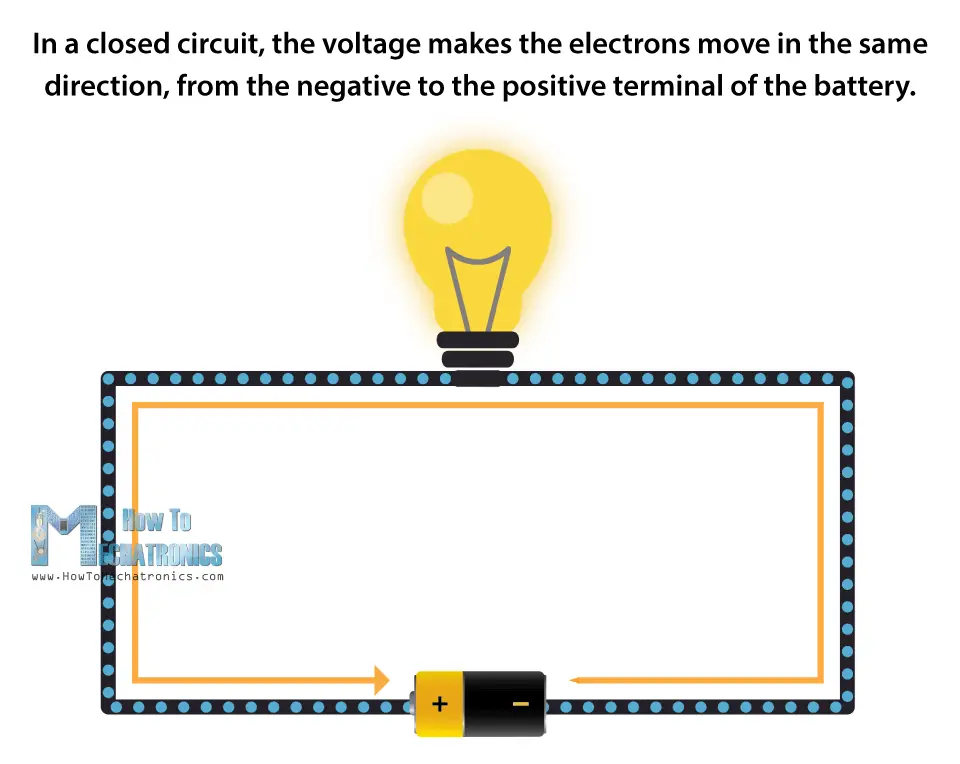
In a closed circuit, the voltage makes the electrons move in the same direction, from the negative to the positive terminal of the battery.
If we add a light bulb to the closed circuit, the electrons will have to pass through it in order to get to the other terminal, thus producing a light.
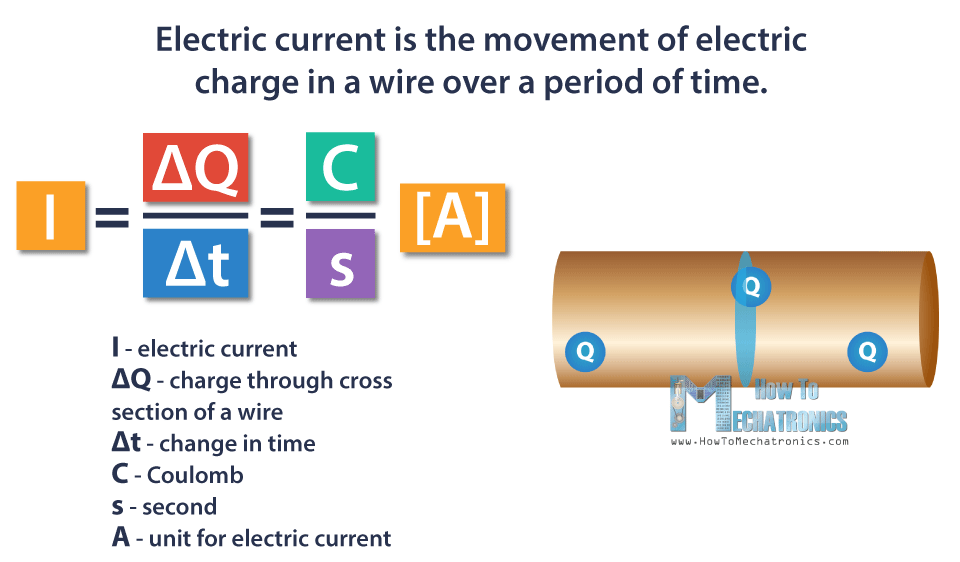
Electric current is the movement of electric charge in a wire over a period of time. The symbol for electric current is I.
One Coulomb of charge passing through a piece of wire over one second is equal to one Ampere of current. Ampere is the unit of measurement of the electric current.
That’s all for this tutorial. In the next Basic Electronics tutorial we will talk about Coulomb’s Law.
I hope you enjoyed this tutorial and learned something new. Feel free to ask any question in the comments section below.